This focus idea is explored through:
Contrasting student and scientific views

Student everyday experiences
Students often have no experience with the idea that some things (particles) can’t be divided. If sand is ground up into tiny pieces it is still sand. There seems no reason why the dividing can’t keep on going to smaller and smaller pieces. This is often described as a
continuous view of matter.
Even when children are introduced to the idea of matter being made of particles, most consider there is still ‘stuff’ between the particles and that something like air fills the empty space. After all, when liquid is poured from a glass so that it is empty it still contains air.
These ideas are further explored in
‘Hannah’s Question,’ a classroom practice vignette.
Research:
Loughran, Berry, Mulhall & Gunstone (2001),
Novick & Nussbaum (1981)
Particles are often assumed to behave in the same way as the substances they make up. For example students can believe that particles themselves can swell, shrink and melt or that the particles in stone are harder than the particles in rubber. Many students consider that ice molecules melt to little droplets of water.
Scientific view
The properties of matter can best be explained using a model in which all materials are composed of tiny particles (atoms,
molecules and
ions).
There is empty space between particles and particles are constantly moving (their speed is changed by temperature). The particles in solids and liquids are quite close to each other, while those in gases are a very long way apart. (There is commonly an increase in volume of at least 1000 fold when substances move from solid or liquid to gas).
Changes of state involving solids, liquids and gases as well as a range of other phenomena can be explained by changes in arrangement and speed of the particles.
Critical teaching ideas
- All matter is composed of tiny indivisible particles too small to see.
- These particles do not share the properties of the material they make up.
- There is nothing in the space between the particles that make up matter.
- The particles which make up matter are in constant motion in all physical states.
Explore the relationships between ideas about the particle theory in the
Concept Development Maps - (States of Matter, Atoms and Molecules, Conservation of Matter, Chemical Reactions, Flow of Energy in Ecosystems, Flow of Matter in Ecosystems)
Students will have a range of explanations for phenomena they have observed. They should move towards the view that a key feature of the particle model is that it was invented by early scientists as a model that explains a wide range of phenomena. Some phenomena can also be explained by a continuous model, but some cannot. Presenting this scientific view through the use of diagrams and models alone has been shown to achieve relatively little conceptual change in students. Essentially the particle model is a way of thinking about matter that requires exploratory discussion to help build student understanding.
A suggested teaching approach is to first ascertain student ideas and explanations, then provide motivating experiences which require explanations that require a particulate view of matter.
If students are going to change their views to the accepted scientific ones, they must firstly become dissatisfied with the usefulness of their existing models. The 'new' scientific conception must be plausible and intelligible and useful in a variety of new situations. This will involve facilitating the exchange of views and challenging students to compare ideas including the scientific perspective.
Finally, provide opportunities for students to test out their new ideas which help to reinforce their usefulness.
Teaching activities
Bring out students’ existing ideas
Asking students to draw what they think they would see if they were ‘shrunk’ to a tiny size and placed in a drop of water will bring out whether or not they understand particles (if they draw particles). Further questioning can elicit from those who do draw particles what they think is between the particles as well as why they drew particles.
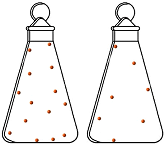
Later on, other probes can bring out if and how students’ thinking has changed. Students can be asked to sketch the particles in a flask of air (Image 1) and then sketch the flask again when half the air has been removed (Image 2). What students draw gives some indication if their thinking has changed.
Encourage students to identify phenomena not explained by the (currently presented) scientific model or idea
Encourage students to ‘challenge the answer’. It is impossible to ‘prove’ the particle model and very difficult to have students develop it from experimental phenomena without a lot of prompting. One approach is to present the standard diagrams of particles in the three states of matter with a few lines of notes on each. Leave out some of what is normally presented, such as comments about
attractive forces, say that this is something that scientists believe and ask students to find phenomena that this does
not seem to explain. Typically this gets them thinking seriously about the model and generates a need for some more features (such as attractive forces).
Promote reflection on and clarification of existing ideas
The initial meanings that students form for the particle model are rarely truly particulate. Provide opportunities for students to articulate the meanings they have and refine these in what is an iterative process. Thought experiments such as imagining a tiny person as small as a needle sticking a needle into a particle of a gas can help students formulate views about particles. Would the gas leak out? Would the gas burst out and make a hissing sound?
Challenge some existing ideas
POE (Predict–Observe-Explain) sequences can challenge students’ thinking on the compressibility of gases and liquids. Ask students to predict how much a syringe full of air can be compressed by one person. Call for justifications, perform the experiment and call for explanations in terms of the particle model. Then repeat with the syringe full of water. This is the more powerful in that many students believe that water will be compressed noticeably, yet it isn’t, and the particle model does explain (and predict) this.
Practise using and build the perceived usefulness of a scientific model or idea
Students come to see the particle model as more useful than a continuous model only gradually, therefore they should observe a range of phenomena which require the particle model to help explain them. Some examples:
- Squeezing syringes containing gas (coloured gas if possible) can show increasing intensity of colour and the idea of particles being pushed closer together.
- Mixing methylated spirits and water can help to show there must be space between particles (50ml + 50 ml = around 97 ml). For this to be effective, consider first adding 50 ml of water to 50 ml of water and measure the volume, and then 50ml of methylated spirits to 50 ml of methylated spirits and measuring the volume. If necessary give a hint when calling for explanations: methylated spirits particles are bigger than water particles.
- Debates and interpretive discussions which encourage students to develop explanations for new situations using the particle theory can help in moving towards a scientific concept of the particle model. For example, ask students to explain why popcorn pops, why we can smell onions at a distance when cooking, and why a syringe containing brown gas appears darker when compressed.
- Challenge students to use their particle model to explain how scents, air fresheners and perfumes are able to spread from the source to all regions in a room.
Clarify and consolidate ideas for/by communication to others
By making a model with plasticine or play dough students represent their understanding of particles and then by drawing on butcher paper students show how the particles may be arranged in each phase. Students can then demonstrate what happens as a substance changes from a solid to a liquid to a gas using the play dough particles.
Role playing different phenomena such as perfume particles when the bottle is open or the particles in melting ice requires students to build a concrete meaning for propositions about particles. Videoing these and debriefing helps pick up ideas not yet assimilated.
Group activities where students construct posters in groups to explain phenomena and present their posters to the class and creative writing where students imagine they are particles in different situations can both provide opportunities for students to discuss and modify their prior views.
Promote reflection on how students’ ideas have changed
Students should be encouraged to reflect on how their views have changed by keeping a log of their views as they experience this topic. Such a learning log is best achieved by creating questions that students can respond to. For example, students can be asked, ‘What are three things that I have learnt today?’ and, ‘How do I think my ideas have changed after today’s lesson?’ When using these sorts of prompts for reflection, it is a good idea to vary the types of questions used.
Research:
Loughran, Berry, Mulhall & Gunstone (2001),
Brook, Briggs & Bell (1983)
Further resources
Science related interactive learning objects can be found on the
FUSE Teacher Resources page.
To access the interactive learning object below, teachers must login to FUSE and search by Learning Resource ID:
-
Types of Matter: solids, liquids gases – students select samples from an outdoor setting and magnify the substances to atomic level so that the particles they consist of can be seen. They then sort the substances into groups based on how the particles are arranged and how they move, classifying the substances as solids, liquids or gases.
Learning Resource ID: HSTCW7