This focus idea is explored through:
Contrasting student and scientific views
Student everyday experiences
 For many students the idea that matter is conserved is not a natural one. They observe that sugar disappears when mixed with water, a large log burns away to a small amount of ash, cars rust and big holes appear, water boils away, frost and condensation appear from apparently nowhere and trees grow apparently from nothing but the soil.
For many students the idea that matter is conserved is not a natural one. They observe that sugar disappears when mixed with water, a large log burns away to a small amount of ash, cars rust and big holes appear, water boils away, frost and condensation appear from apparently nowhere and trees grow apparently from nothing but the soil.
It may seem to students that matter disappears or appears during processes such as dissolving, burning, evaporation, boiling, rotting, respiration, rusting, condensation and growth of plants. Invisible gases are involved in many of these processes leading to many of these alternative student conceptions.
Research: Driver (1985), Russell, Harlen & Watt (1989)
Students also often believe that matter is exchanged or converted into energy (for example, they believe that wood turns into heat during combustion and food turns into energy when we metabolise it) or they confuse the energy of food (listed on packets as kilojoules) with the weights of the listed ingredients. Students also often believe that the sun’s energy is changed into plant matter during the process of photosynthesis.
The process of evaporation may also challenge students’ conceptions as many students believe that substances become lighter if they change into the gas state. If a liquid evaporates inside a sealed container then they believe that the combined weight of the container and the liquid will be reduced by the weight of the liquid because it has apparently disappeared.
Research: Stavy (1990)
Although at this level the majority of students will have an understanding of the particle nature of atoms, for many the numbers of atoms are not conserved during chemical reactions. For example, the number of atoms appears to grow in the bark of trees, and their numbers drop during processes such as combustion or decay and increase during photosynthesis.
Scientific view
The idea of indivisible atoms helps to explain the conservation of matter. If the number of atoms stays the same no matter how they are rearranged, then their total weight stays the same.
In all physical and chemical changes, the total number of atoms remains the same, hence when substances interact with one another, combine or break apart, the total weight of the system remains the same.
Growing plants obtain their new carbon (the great majority of their dried weight) from carbon dioxide i.e. from the air. When we lose weight by dieting or exercise, most of the loss is from breathing out the carbon atoms metabolised from our fat as carbon dioxide. When a liquid evaporates in a sealed container, the weight stays the same; the gas particles are affected by gravity in the same way as tennis balls and they consequently hit the bottom surface of the container with more force than they hit the top.
Albert Einstein’s prediction that mass can be converted into energy has been experimentally validated by numerous nuclear experiments. A consequence of this is that the statement, “the total weight of a system remains the same” is more correctly only a very good approximation for all non nuclear changes.
Critical teaching ideas
In physical and chemical changes:
- particles just don't disappear or get created, rather their arrangements change
- in any change involving matter, all of the matter must be accounted for. Matter does not turn into or appear from energy
- particles are rearranged to create substances different from the original
- there is no change in weight when substances move in and out of the gas state.
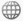 Explore the relationships between ideas about conservation of mass in the
Concept Development Maps - (Conservation of Matter, States of Matter, Flow of Matter in Ecosystems)
Explore the relationships between ideas about conservation of mass in the
Concept Development Maps - (Conservation of Matter, States of Matter, Flow of Matter in Ecosystems)
In your teaching of conservation of matter and hence weight, students need to be encouraged to change their views from those based on their everyday experiences to more scientific views such as the idea that there are only a fixed number of particles in the world and these building blocks are continually being rearranged into new things.
This is a difficult, abstract idea and we can use analogies to assist students with understanding. For example: ‘It’s a bit like having a bucket of Lego: you can build lots of different things but you only have a certain number of Lego pieces to do it with'. One of the difficulties is that closed systems involving changes such as combustion and respiration are almost impossible to set up and weigh in a classroom. You can disprove by experiment only a few of the common alternative conceptions in this area.
Research: Loughran, Milroy, Berry, Gunstone & Mulhall (2001)
It is important to discuss a variety of change situations that appear to involve non-conservation of matter and to revisit this issue in other topics such as ecosystems, food and diet, and sources of energy. Students should be encouraged to make predictions about these processes in a supportive classroom environment where they can be assisted to develop new theories, critically analyse their understandings and those of others, and compare these with scientific views presented by the teacher. It is easier to try to eliminate the alternative conceptions that can be experimentally tested first and have students then come to an understanding of the scientific model as one which can best explain a wide range of phenomena.
Reflection by students on how their views have altered will be a valuable component of achieving long lasting conceptual change in students and an understanding of the scientific explanations.
Research: Loughran, Milroy, Berry, Gunstone & Mulhall (2001)
Teaching activities
Students should both discuss and observe phenomena where there is an apparent change in weight and be encouraged to seek alternative explanations.
Promote reflection on and clarification of existing ideas and allow students to see how their ideas have changed
Ask students to write down and keep their views on a range of situations like the following:
- A fresh corpse is sealed in an airtight dungeon. 100 years later, the corpse is now a skeleton. Does the total mass of the dungeon, the corpse and other contents increase, decrease or remain unchanged? Where does the flesh go?
- Imagine a pile of firewood burns in a sealed room. Does the total mass of the room and its products increase, decrease or stay the same? (See the tree growing in a box, below).
Open up discussion via a shared experience and promote reflection on and clarification of existing ideas
POE (Predict -Observe-Explain) activities can be valuable in both eliciting student prior views and leading to reframing of these concepts.
Ask students to predict what will happen when materials (such as steel wool or magnesium) are burnt. Students should then weigh materials before and after burning, while ensuring all products of combustion are retained. Note that these experiments do not prove that the total weight is conserved in a closed system. In this case the system is open and the total weight of the steel wool/magnesium is increased as they combine with oxygen from the air. What these experiments do show convincingly is that weight is not always lost during combustion, and hence the notion of matter turning into heat is not sustainable.
Similarly, explore what happens in evaporation. Weigh a tightly stoppered flask containing 1 - 2ml of acetone, warm it slowly and then reweigh. (Students often predict the weight of the flask has decreased because gases are lighter than liquids, or the molecules are no longer sitting on the bottom.)
Promote reflection on and clarification of existing ideas
Interpretive discussions (where a teacher delays judging ‘incorrect’ comments) can allow students to consider and reframe their views (after viewing the changes above and the many others that can be devised).
Clarify and consolidate ideas for/by communication to others
Based on the activities above, small groups of students could make posters and present them to the class to communicate their ideas and explanations.
Open up discussion via a shared experience and promote reflection on and clarification of existing ideas
Student could weigh several antacid tablets and a small soft drink bottle filled with water separately to determine their combined weight. The tablets are then added to the water and the bottle quickly sealed and reweighed after the tablets have fully dissolved. The ‘before’ and ‘after’ weights should be similar, even though the tablets have completely disappeared and a gas was generated. Encourage students to discuss the results and make some judgement about how these results are consistent with or challenge their views about the conservation of matter.
Promote reflection on and clarification of existing ideas
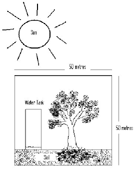
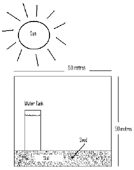
Another problem which tests students’ understanding of the theory of conservation of matter is the following: inside a large sealed glass box, there is plenty of moist soil, a water tank with drip irrigation and the seed of a fast growing tree. The box is placed in the sun for a year and a tree grows inside the box. The students are asked if the total weight of the box and the contents will increase, decrease or remain unchanged during the year’s growth of the tree.
| Teaching activities - Understanding the theory of conservation of matter |
|---|
|
Image 1 shows the tree beginning as just a seed. |
|
Image 2 shows that one year later a tree has grown. |
A further variation to consider is: without opening the box the tree is set on fire. After burning, will the weight of the box change? What will happen to the number of atoms before and after both these changes?
Clarify and consolidate ideas for/by communication to others
Ask students to consider the following situation: your hand is composed of carbon atoms (contained in protein molecules). Consider one of these carbon atoms, let's call him ‘Gilbert’. Suppose that one million years ago Gilbert was in a blade of grass. Write a possible history of Gilbert over the last million years. In your answer refer to as many processes as you can.
When you present this task, warn students they are likely to revert back to their prior views. (Very few students manage to write this story without drawing on their prior views and writing things which are in conflict with what they have just been taught. A common error is to return to beliefs that plants draw most of the material needed for growth from the soil, with Gilbert repeatedly being returned to the soil as a consequence of the decay of dead organic material and then drawn from the soil by another plant). For students who return with stories showing very little knowledge of what they have just learnt, see if they can explain why they wrote the stories in this way.
Promote reflection on how students’ ideas have changed
Ask students to look again at their views on the scenarios involving decay and combustion and reflect on whether they changed their views. If they have, ask them how they would change their answers to the situations and why they would change them. (Never assess their initial responses, but one can assess the reflective responses the second time around).
Students could also formulate their own hypotheses about weight changes in burning, respiration and photosynthesis and conduct investigations in order to prove or disprove them.
Further resources
Science related interactive learning objects can be found on the
FUSE Teacher Resources page.
To access the interactive learning object below, teachers must login to FUSE and search by Learning Resource ID:
-
Chemical Reactions: Combustion – students investigate chemical reactions at a molecular level. They break apart molecules of oxygen and another substance into their component atoms and rearrange them to make molecules of new substances. For example, they explore how methane burns in oxygen to produce carbon dioxide and water. They also work out the number of molecules of each substance needed to balance the chemical equation
Learning Resource ID: HY5GUN