This focus idea is explored through:
Contrasting student and scientific views
Student everyday experiences
 Students of all ages have difficulty in understanding that matter is composed of particles and despite the best teaching efforts often hold on to the understanding that matter is continuous until well into their secondary education or beyond. A constant difficulty for students is an appreciation of just how small atomic particles are. Students often consider that bacteria or cells are so small that they must be of a comparable scale to the size of atomic particles.
Students of all ages have difficulty in understanding that matter is composed of particles and despite the best teaching efforts often hold on to the understanding that matter is continuous until well into their secondary education or beyond. A constant difficulty for students is an appreciation of just how small atomic particles are. Students often consider that bacteria or cells are so small that they must be of a comparable scale to the size of atomic particles.
Research: Nussbaum (1985), Berkheimer, Charles, Okhee & Theron (1988), Nicoll (2001)
This lack of appreciation of the minute size of particles often leads students to attribute the atomic particles with similar macroscopic properties to the substance they are part of. For example, students may believe that liquids are composed of liquid particles; if a tiny pin was used to puncture one of the particles, liquid would emerge. This idea is sometimes held even more strongly by some students who may believe that atomic particles mimic macroscopic properties such as colour, hardness and physical state. For example, they may believe that blue coloured solids must be composed of hard blue particles, water particles will expand as they freeze and particles will get smaller as they evaporate.
Research: Gomez & Pozo (2004)
Throughout their education students are presented with a confusing array of models of atoms based on ideas from orbital planetary motion to images of constituent particles resembling either static or moving ‘hard’ balls. The more closely students examine the models the more they appear to contain more and more particles. Even high achieving students appear to find these attempts at creating an age appropriate conceptual and scientific model of the atom confusing.
Research: Harrison & Treagust (1996), Nicoll (2001)
Scientific view
Most macroscopic properties of a substance are a consequence of how the constituent particles are arranged and held together. For example, graphite and diamond both contain only carbon atoms. In one substance they are held together in sheets or layers so they slide easily over one another making it useful as a dry lubricant. In the other form they are held together with strong bonds so they create the hardest natural substance known. This illustrates that the constituent particles of a substance do not have the same physical properties as the substance.
All symbolic representations of atomic particles have limitations. In particular, matter is not composed of tiny hard balls although this model can be useful at junior levels.
Critical teaching ideas
The creation of models is an important aspect of teaching in this area if we are to facilitate student understanding. The notion of atoms, molecules, protons, electrons and neutrons remains abstract and sits entirely outside our experiences.
- We create models to explain the behaviour, (but not the appearance) of these microscopic particles.
- We create multiple models as no one model will explain all the behaviours simultaneously. Sometimes it is helpful to describe atoms as hard, perfectly elastic balls, and sometimes we describe them as sticky fuzzy balls.
- When using multiple models, it is important to critique each model.
- Any property of a material is a result of the arrangement of the particles, not a result of the individual particles having that property.
 Explore the relationships between ideas about atomic structure in the
Concept Development Maps - (Atoms and Molecules, Chemical Reactions, States of Matter)
Explore the relationships between ideas about atomic structure in the
Concept Development Maps - (Atoms and Molecules, Chemical Reactions, States of Matter)
Teaching activities
Bring out existing ideas
Ask students to draw what they think an atom looks like. Their representations will vary, but many will draw images with similar features to those shown below. Discuss how these representations have originated and how they are purposefully used to demonstrate particular features of atomic properties.
For example, the images below are designed to illustrate particular features:
| Bring out existing ideas |
|---|
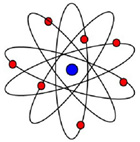
| - Image 1 shows an early naïve model of an atom designed to illustrate the inner nucleus and the orbiting electrons.
|
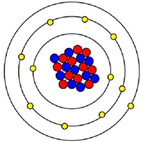
| - Image 2 emphasises the presence of protons and neutrons in the nucleus and electrons orbiting in surrounding concentric shells.
|
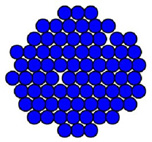
| - Image 3 illustrates the intermolecular forces holding the spherical atoms together.
|
| - Image 4 shows the presence of chemical bonds between the atoms in a complex molecule.
|
It can be helpful to look at these images and ask students to identify how the models are NOT like real atoms. This will encourage the students to reflect on their existing understandings and see where they may need improvement.
Practise using and build the perceived usefulness of a scientific model or idea
A model proposed by Nobel laureate, Richard Feynman, has recently found increasing support. The model proposes that the atom has many properties similar to a sticky, fuzzy ball. This model can be used to demonstrate many of the observed behaviours of atoms and is argued to be more useful to students than many of the existing alternate models such as the ‘hard billiard ball’ or the planetary orbital models.
Key aspects of the model:
- it can be modelled easily by use of a soft foam ball or computer drawing software
- its stickiness naturally leads to the idea of molecule formation and different states of matter
- the property of fuzziness and stickiness allows consistency with more complex models.
Research: Wright (2003), Bucat (1983)
Importantly this model is seen as remaining useful to students. This naïve image remains consistent across age appropriate development and has the potential, in later years, to support more complex understandings of the atom (for example, with regard to quantum mechanics or electron clouds). One of the many challenges in teaching this content area is the demands it places on students to switch between using one symbolic representation of atomic particles to another. For students, knowing and understanding the limitations that are inherent with each model are important for determining their usefulness .
Research: Wright (2003)
The idea that atoms can be considered to behave as though they are ‘sticky’ provides a naïve explanation for their apparent willingness to attach themselves to each other to form more complex molecules. This will also assist in understanding their behaviour as substances change state. Encourage students to discuss what they see to be the advantages and the disadvantages of this model. Consider various changes of state and encourage the students to describe the formations of the atoms and how they might interact with one another to achieve the macroscopic properties they observe.
Explore the difference between thermosetting and thermoplastic polymers, which have essentially the same building blocks, but very different properties. These properties can be explained in terms of their chain arrangement. (The extent of cross linking between chains is the major difference here, and how it influences the flexibility of the structure). For example students could compare nylon, a thermoplastic, with
Araldite which is thermosetting.
Open up discussion via a shared experience
Students can investigate the molecular work bench website:
Have students investigate the range of atomic simulations representing substances in different states and encourage them to discuss how the observed movements of the individual atomic particles contribute to the observed properties of the substance.