This focus idea is explored through:
Contrasting student and scientific views
Student everyday experiences
Students have particular problems with recognising the existence of gases as a physical state of matter because most gases are invisible. The notion of ‘seeing is believing’ is very important to young students and it has a powerful influence on their ability to form understandings. An added difficulty is that there are almost no everyday occurrences in the students’ world of coloured gases that can be observed.
Research:
Stavy (1988)
A common belief encouraged by the invisibility of gases is they are weightless and do not occupy space . Confusion can originate when students are regularly told that containers and jars with nothing in them are empty but are then expected to grasp that although empty, they really contain air.
Research:
Russell, Longden & McGuigan (1991)
A third problem is that the word gas when used in everyday language has other meanings such as natural gas and camping gas that are not helpful to students in shaping their understanding of the term as a classification of a physical state of matter.
These issues are also addressed in the focus idea
Introducing scientific language.
Scientific view
The terms ‘solid’, ‘liquid’ and ‘gas’ are three simple but useful classifications of the states in which matter can occur.
These are not the only states of matter used by scientists and their use is not without problems; for example, how do we classify substances like hair gel, grease or mayonnaise?
This issue is also addressed in the focus idea
Problems with classification.
All gases have weight (1 litre of air in a normal room weighs approximately 1 gram). Most gases encountered by students are colourless (e.g. air, water vapour and oxygen). Smoke, fog and smog are not coloured gases but rather small particles of carbon or water droplets suspended in the air. Some gases are visible (e.g. nitrogen dioxide and chlorine gas) but these are rarely encountered by students.
Critical teaching ideas
- In addition to solids and liquids, gases are also a physical state in which matter can occur.
- All gases have weight.
- Unlike solids and liquids, gases will occupy the entire container that encloses them.
 Explore the relationships between ideas about gases and states of matter in the
Concept Development Maps - (Conservation of Matter, States of Matter)
Explore the relationships between ideas about gases and states of matter in the
Concept Development Maps - (Conservation of Matter, States of Matter)
At this level it is appropriate to encourage students to build an understanding that matter can be classified as a solid, liquid or gas and that these terms describe the appearance of substances and how substances behave. It is important that the gaseous state is not ignored when performing simple classification tasks.
As students are consolidating their understanding of these terms, avoid presenting them with substances that are difficult to classify. These will become apparent to students as their understanding of each state crystallises and they begin to realise the boundaries are sometimes ‘fuzzy’.
These issues are also addressed in the focus idea
Problems with classification.
It is more difficult to convince students that gases have weight because it takes a lot of gas for the weight to become noticeable. Some students may see the ability of high winds to move a heavy object as convincing but this is not always helpful to students. Blowing air through a straw directed at a ball of crumpled paper is one way to demonstrate that something must be pushing on the paper to make it move even though it can’t be seen.
An everyday experience that is more powerful is the change in weight when filling a propane gas bottle. Large bottles are clearly heavier after filling. At this level it is not appropriate to discuss the propane being stored as a liquid under pressure or changing state from a liquid to a gas as it is released from the bottle.
Teaching activities
Open up discussion via a shared experience and collect evidence and data for analysis
For a simple activity to demonstrate the movement of gases around a room, firstly position students evenly around the classroom. Release a two second spray from a pressurised spray room deodoriser in one corner of the room. Invite the students to raise their hands as soon as they detect the smell as it moves around the room. It should only take a short time before all the students can detect the smell of the gas as it spreads. This gas movement will be faster on a hot day. Invite students to play a game of seek and identify the smell, using a couple of different room deodoriser spray cans.
Provide an open problem to be explored via play and challenge some existing ideas
 To encourage students to explore the idea that gases occupy space, ask them to plan a rescue mission for a stricken submarine. Students can decorate a simple clear plastic cup to be the submarine that is running out of air for its crew. Fill this cup almost completely with water and invert it so it is largely above the surface and its rim is below. Using another clear plastic cup invite students to capture air and transfer it to the stricken boat so the crew can breath. How will they do this? Let them take turns to pour the air from the second cup into the submarine. Discuss how air occupies a constant space and can be enclosed in the cup and allowed to rise into the cup above pushing the water out of the space.
To encourage students to explore the idea that gases occupy space, ask them to plan a rescue mission for a stricken submarine. Students can decorate a simple clear plastic cup to be the submarine that is running out of air for its crew. Fill this cup almost completely with water and invert it so it is largely above the surface and its rim is below. Using another clear plastic cup invite students to capture air and transfer it to the stricken boat so the crew can breath. How will they do this? Let them take turns to pour the air from the second cup into the submarine. Discuss how air occupies a constant space and can be enclosed in the cup and allowed to rise into the cup above pushing the water out of the space.
Clarify and consolidate ideas for/by communication to others
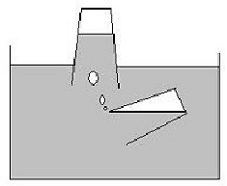 Invite the students to write a brief story about the submarine rescue mission. Encourage them to draw pictures or simple diagrams of the cups and where they needed to be to pour the trapped air from the cup into the submarine. Discuss how the crew felt when they were able to breathe the fresh air supplied by the rescue mission.
Invite the students to write a brief story about the submarine rescue mission. Encourage them to draw pictures or simple diagrams of the cups and where they needed to be to pour the trapped air from the cup into the submarine. Discuss how the crew felt when they were able to breathe the fresh air supplied by the rescue mission.