This focus idea is explored through:
Contrasting student and scientific views
Student everyday experiences
 At this level, students are expected to 'explain the behaviour and properties of materials in terms of their constituent particles and the forces holding them together’ (VELS standards Level 6). However, the fact that students may be able to draw the usual static arrangements of particles in solids, liquids and gases does not mean that they hold a fully particulate view of matter. Research evidence suggests that many students at this age and older still hold a number of alternative conceptions about particles which prove difficult to extinguish. They often lack an appreciation of the very small size of particles, attribute macroscopic properties to microscopic particles, have difficulty appreciating the motion of particles in all states of matter and have problems understanding forces between particles.
At this level, students are expected to 'explain the behaviour and properties of materials in terms of their constituent particles and the forces holding them together’ (VELS standards Level 6). However, the fact that students may be able to draw the usual static arrangements of particles in solids, liquids and gases does not mean that they hold a fully particulate view of matter. Research evidence suggests that many students at this age and older still hold a number of alternative conceptions about particles which prove difficult to extinguish. They often lack an appreciation of the very small size of particles, attribute macroscopic properties to microscopic particles, have difficulty appreciating the motion of particles in all states of matter and have problems understanding forces between particles.
Research: Driver (1987)
Many students who appreciate that matter is particulate still retain some former views and consider that particles can change their form (solid to liquid), explode, burn, expand, change shape and colour or shrink. Students visualise atoms, molecules and ions to be little ball-like objects (perhaps because of the way the information has been presented) and this contributes to them confusing the properties of the particles with the macroscopic nature of the materials that they make up.
Research: Happs (1980)
These ideas are also explored in the focus idea
Macroscopic and microscopic properties.
Students frequently fail to understand the dynamic nature of particles; they tend to think of them as static. Students may believe that gas particles are moving slowly in ways similar to what they observe when they see suspended dust particles in a beam of light. Random particle motion in liquids and gases is a difficult concept for students to appreciate. When asked, “Why don't gas particles fall to the bottom of a vessel?” only about 50% of students thought that the particles were in constant motion. Students stated that particles were forced apart (by heat acting as a substance) when gases were heated. When gases condensed to a liquid, many students attributed this to increased attractive forces between particles.
Research: Novick & Nussbaum (1981)
Students frequently find it difficult to appreciate particle movement in solids and this leads to different conceptions about freezing and melting. Some examples of students’ thinking about the behaviour of particles in a melting ice block are:
Student 1: “The particles start to break away from each other because of the rise in temperature. When they have broken away from each other, they turn from a crystal form to a solution form."
Student 2: "When a block of ice is taken out of a freezer, the sudden change of temperature reacts on the particles making them decrease in size."
Scientific view
Atoms are incredibly small and cannot be seen with even the most powerful light microscope. We use multiple models of atoms to help explain chemical processes and describe their behaviour.
In gases the particles move rapidly in all directions, frequently colliding with each other and the side of the container. With an increase in temperature, the particles gain kinetic energy and move faster. The actual average speed of the particles depends on their mass as well as the temperature – heavier particles move more slowly than lighter ones at the same temperature. The oxygen and nitrogen molecules in air at normal room temperature are moving rapidly at between 300 to 400 metres per second. Unlike collisions between macroscopic objects, collisions between particles are perfectly elastic with no loss of kinetic energy. This is very different to most other collisions where some kinetic energy is transformed into other forms such as heat and sound. It is the perfectly elastic nature of the collisions that enables the gas particles to continue rebounding after each collision with no loss of speed. Particles are still subject to gravity and hit the bottom of a container with greater force than the top, thus giving gases weight. If the vertical motion of gas molecules did not slow under gravity, the atmosphere would have long since escaped from the Earth.
In liquids, particles are quite close together and move with random motion throughout the container. Particles move rapidly in all directions but collide with each other more frequently than in gases due to shorter distances between particles. With an increase in temperature, the particles move faster as they gain kinetic energy, resulting in increased collision rates and an increased rate of diffusion.
In a solid, the particles pack together as tightly as possible in a neat and ordered arrangement. The particles are held together too strongly to allow movement from place to place but the particles do vibrate about their position in the structure. With an increase in temperature, the particles gain kinetic energy and vibrate faster and more strongly.
The attractive force in solids need not be stronger than in liquids or gases. For example the forces between solid helium particles (at -270 degrees C) are still very weak. By comparison, the forces between iron vapour particles (requires very high temperatures) are very strong. If you compare different substances that are at the same temperature, then the average kinetic energy of the particles will be the same (i.e. if the particles have the same mass then they will move with the same speed), but the attractive forces in solids will be greater than those in liquids, which will be greater than those in gases. Attractive forces don't get weaker when a substance moves from the solid to the liquid to the gas state, rather the kinetic energy of the particles increases (implying faster motion), allowing them to overcome the attractive forces.
Critical teaching ideas
- All matter is made up of atoms which are far too small to see even with the most powerful light microscopes.
- Particles in all states of matter are in constant motion and this is very rapid at room temperature. A rise in temperature increases the kinetic energy and speed of particles; it does not weaken the forces between them.
- The particles in solids vibrate about fixed positions; even at very low temperatures.
- Individual particles in liquids and gases have no fixed positions and move chaotically.
- The collisions between particles differ from collisions between macroscopic objects in that they are perfectly elastic: i.e. the kinetic energy of the particles remains constant and no energy is transformed into other forms during collisions.
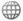 Explore the relationships between ideas about movement of particles in the
Concept Development Maps - (Chemical Reactions, States of Matter)
Explore the relationships between ideas about movement of particles in the
Concept Development Maps - (Chemical Reactions, States of Matter)
Students at this level have been exposed to ideas about particles (including atoms, ions and molecules) on a number of occasions, yet many of them retain alternative or naïve views about the nature of particles and these can inhibit their understanding. Aim to adopt teaching strategies that promote dissatisfaction in students with their existing ideas, and promote a scientific conception that is plausible, consistent and useful in a variety of situations.
Teaching activities
Bring out students’ existing ideas
It is important to ascertain the majority of students' prior views at the commencement of teaching to establish their existing understanding of the particle model of matter.
Ask students for their ideas about the size of atoms compared with other small things such as cells, bacteria and viruses. This can be done by asking them to draw the relative size of these on the same scale (a scale where a human cell is the size of a page or poster). Bring out the idea that atoms are so much smaller again. Look for other activities that can help reinforce the idea that particles are very, very small.
Show students the conventional drawings of particles in solids, liquids and gases and ask them if and how fast they think they are moving.
Challenge some existing ideas
A number of the issues raised in the focus idea ‘Conservation of mass’ are relevant here and the weighing of a flask containing a small amount of acetone before and after evaporation can be used to challenge students’ ideas about matter being lighter in the gas state and to raise problems with the static drawings of gas particles in texts. For more information see:
Conservation of mass.
Help students work out some of the ‘scientific’ explanation for themselves
With a little encouragement, a class can usually work out by discussion that the particles in gases must be hitting the bottom of the flask harder than the top and hence that they are affected by gravity. This can be extended to explaining why the Earth’s atmosphere thins and eventually ends – the upward vertical motion of the particles ceases.
Promote reflection on and clarification of existing ideas and encourage students to identify phenomena not explained by the (currently presented) scientific model or idea
As particles cannot be directly observed, much of the teaching involves looking for apparent problems or inadequacies with the sorts of static pictures of particles given in earlier years. Encourage students to identify these and talk through possible explanations. Some prompts:
- What holds air particles up?
- Are air particles moving faster on a windy day?
- How can gases have weight?
- Why don’t air molecules fly off into outer space?
If needed, raise issues such as these, which will open up discussion, but it is better if the students themselves come up with some. Note that many of the issues are to do with gases – it is their properties that we most need a particulate model to explain.
To reinforce the notion of elastic collisions, ask what would happen if collisions between gas particles were not elastic. What practical consequences would there be for people? This can be introduced by dropping different types of balls (such as a soccer ball, a table tennis ball and a bouncy ball (from toy shops)) and explaining that a bouncy ball behaves more like gas particles.
Open up discussion via a shared experience
Using activities like POE (Predict-Observe-Explain) can help students think about and then question their existing ideas. The following activity will help students consider their ideas about the movement of particles.
Set up two pairs of flasks each connected by a valve (see diagrams below). Both pairs have brown nitrogen dioxide in the left hand side flask.
| POE (Predict-Observe-Explain) experiments |
|---|
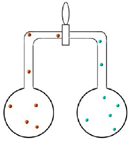
| The first pair also has air in the right hand side flask. Students are asked to predict what will happen when the valve between the two flasks is opened. The brown colour will spread very slowly from one flask to the other because the particles have frequent collisions with the air particles. |
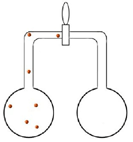
| The second pair of flasks has brown gas in the left hand side flask but the right hand side flask is completely evacuated. Students are asked again to predict what happens when the valve is opened. The very fast speed of the molecules means that they fill the evacuated flask very quickly. |
 Diffusion experiments can reinforce the idea of movement of particles. These can also be used as POEs.
Diffusion experiments can reinforce the idea of movement of particles. These can also be used as POEs.
For example:
- a crystal of copper sulphate is placed in agar gel; the blue colour slowly diffuses through the gel
- a potassium permanganate crystal is placed in a glass and water is slowly added. See the image. Alternatively water is very slowly added to a solution of potassium permanganate in a burette.
Brownian motion can also be observed using stereo microscopes when sulphur powder or camphor is sprinkled on the surface of water or ethanol.
Practise using and build the perceived usefulness of a scientific model or idea
A cotton wool piece soaked in ammonia is placed at one end of a long glass tube with another soaked in hydrochloric acid (HCl) placed at the other end. Eventually a white ring will form where the two gases meet. The two gases are at the same temperature and thus the particles have the same kinetic energy; the ring forms closer to the source of heavier and thus slower moving HCl. This is predicted by a comparison of the relative molecular masses. Including a strip of universal indicator paper in the tube allows the gas diffusion to be tracked. This is an example of a POE where it is useful to draw students’ attention to a relevant piece of science before they make their prediction as it builds usefulness for the concept of relative molecular mass (Mr values).
Students need to be given the opportunity to use the scientific conceptions about particle theory in other settings. Ask students to observe and then explain the changes in terms of particle movement in scenarios such as melting wax or plastic, mothballs (naphthalene) vanishing in a cupboard and the scent of perfume spreading through a room.